Mullvad Leta
Service shutdown notice
Leta has been permanently shut down as of November 27, 2025. Read more.
If you have Leta set as the default search engine in your browser, we recommend switching to DuckDuckGo.
How to set DuckDuckGo as the default search engine in Mullvad Browser
 Click the top right menu icon (3 horizontal lines).
Click the top right menu icon (3 horizontal lines). In the bottom part of the dropdown menu, click on "Settings".
In the bottom part of the dropdown menu, click on "Settings".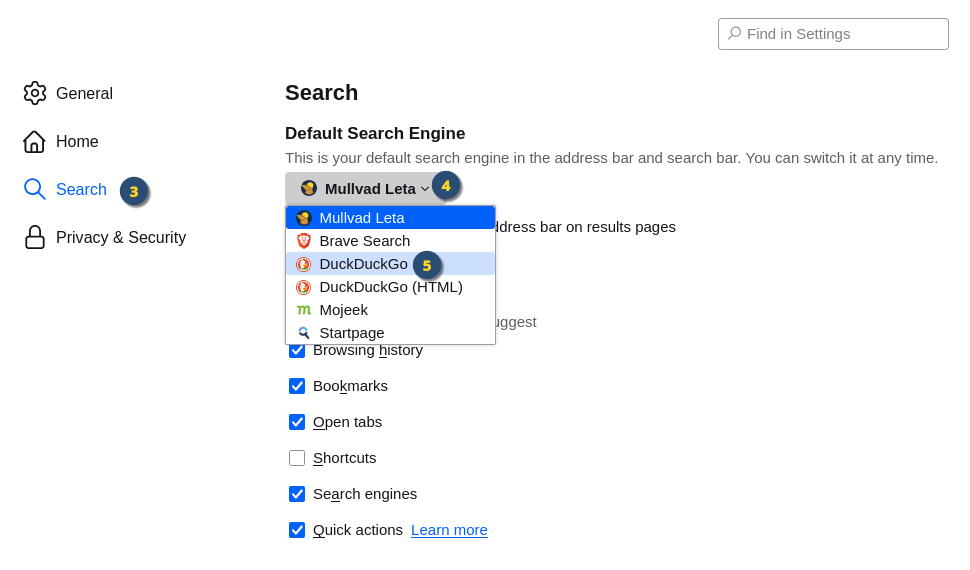 In the "Settings" page, click on "Search" in the left side menu.
In the "Settings" page, click on "Search" in the left side menu. Click on the dropdown to view the list of search engines.
Click on the dropdown to view the list of search engines. Click on "DuckDuckGo" to set DuckDuckGo as your default search engine.
Click on "DuckDuckGo" to set DuckDuckGo as your default search engine.